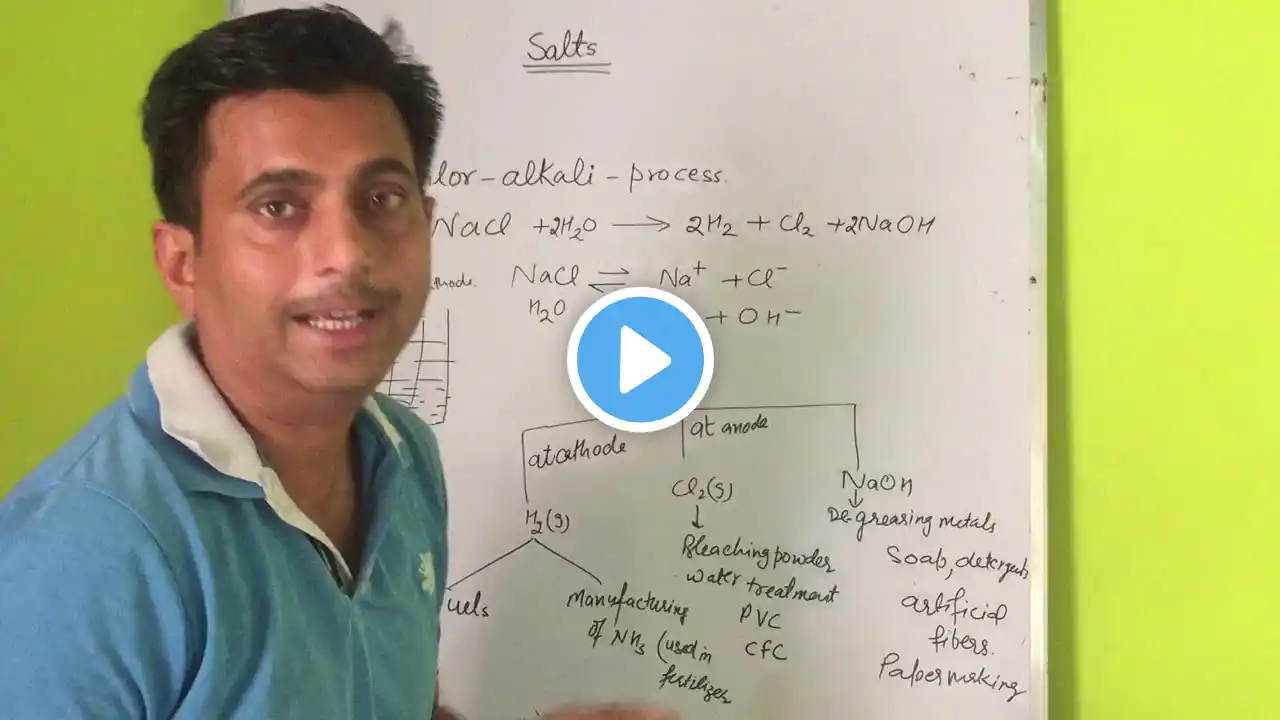
Chlor-Alkali Process #shorts
Chlor-Alkali Process The chlor-alkali process is an industrial process that produces chlorine and sodium hydroxide (caustic soda) from sodium chloride (common salt) through electrolysis. The process involves the electrolysis of a sodium chloride solution, resulting in the production of chlorine gas at the anode and sodium hydroxide and hydrogen gas at the cathode. Chemical Reactions: 1. Anode (Oxidation): 2Cl^- → Cl2 + 2e^- 2. Cathode (Reduction): 2H2O + 2e^- → H2 + 2OH^- Products: 1. Chlorine (Cl2): Used in various industries, such as water treatment, pharmaceuticals, and plastics production. 2. Sodium Hydroxide (NaOH): Used in industries like paper, textiles, and soap manufacturing. 3. Hydrogen (H2): Can be used as a fuel or in various industrial processes. The chlor-alkali process is a crucial industrial process that produces essential chemicals for various applications. #shorts #experiment #experimentshorts