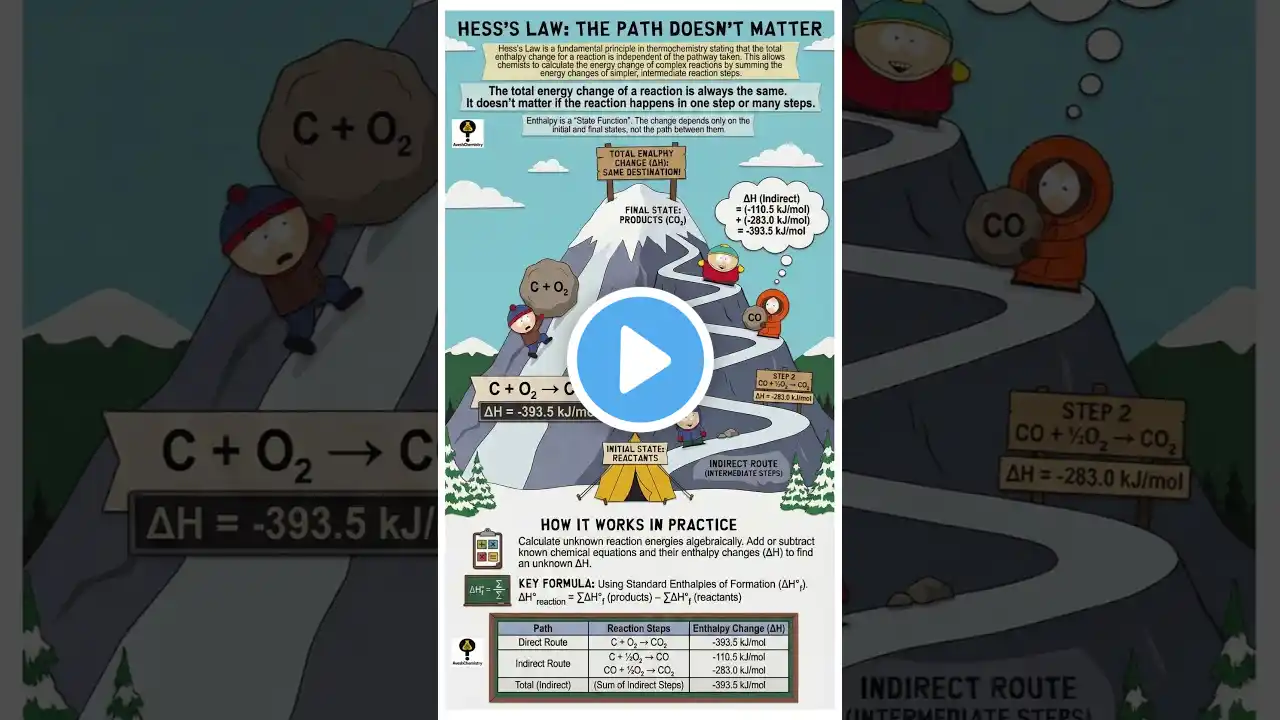
Why Hess’s Law is a Cheat Code for Chemistry ✍️
Confused about Enthalpy change? Hess’s Law of Constant Heat Summation states that the total enthalpy change for a reaction is the same, regardless of the path taken! $\Delta H_{total} = \Delta H_1 + \Delta H_2 + \Delta H_3$ Perfect for JEE Main & NEET quick revision. Subscribe for more 1-minute Chemistry hacks! #HessLaw #Thermodynamics #ChemistryShorts #JEE2025 #NEETPreparation #Class11Chemistry #ExamHacks